 We
talk about acidity and alkalinity of the soil but often
don't have a good handle on exactly what that means. Of
course, these terms do not just apply to our soil. They
are used to describe any chemical compound.
We
talk about acidity and alkalinity of the soil but often
don't have a good handle on exactly what that means. Of
course, these terms do not just apply to our soil. They
are used to describe any chemical compound.
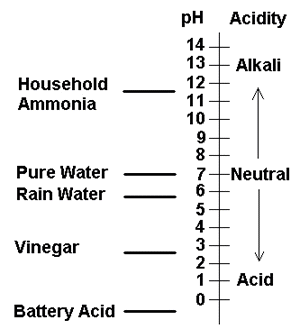
Technically, pH is a
measurement of the concentration of hydrogen ions
present. It is ranked on a 14 point scale with 7.0 being
neutral i.e. neither acid nor alkaline. If the pH number
is lower than 7.0 it is said to be acidic while a number
higher than 7.0 is said to be alkaline or base.
The pH scale is what is
called logarithmic meaning that each change of 1 unit of
pH means a 10 times change. So, a pH of 6.0 is ten times
more acid than one of 7.0. A pH of 5 is 100 times as
acidic as one of 7.0.
Most landscape plants do
best with a pH in the slightly acid range between 6.0
and 7.0. A handful of "acid loving" plants such as
rhododendron, azaleas,
boxwood,
blueberries and
pin oak
need a more acid environment between 4.5 and 5.5. A
handful of species, especially those from the Western
part of the
United States
do best in an alkaline soil.



